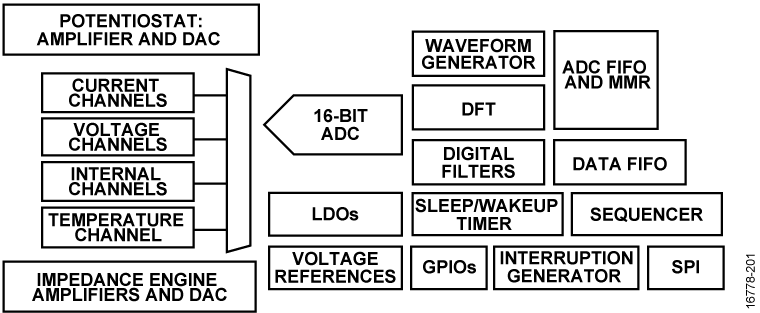
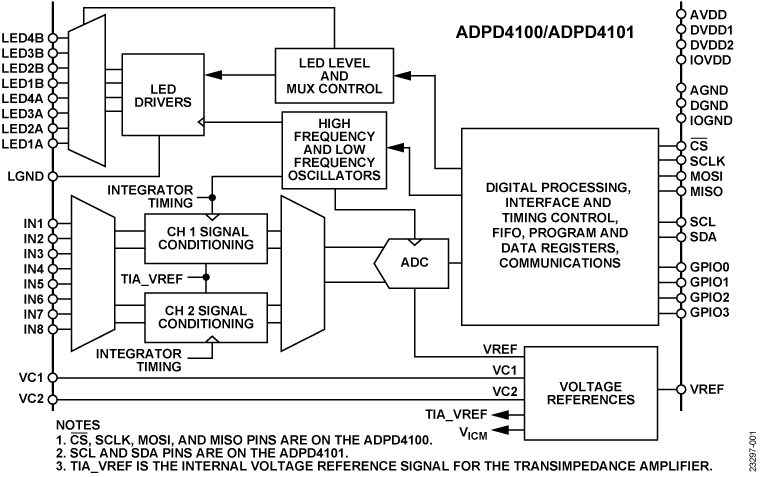
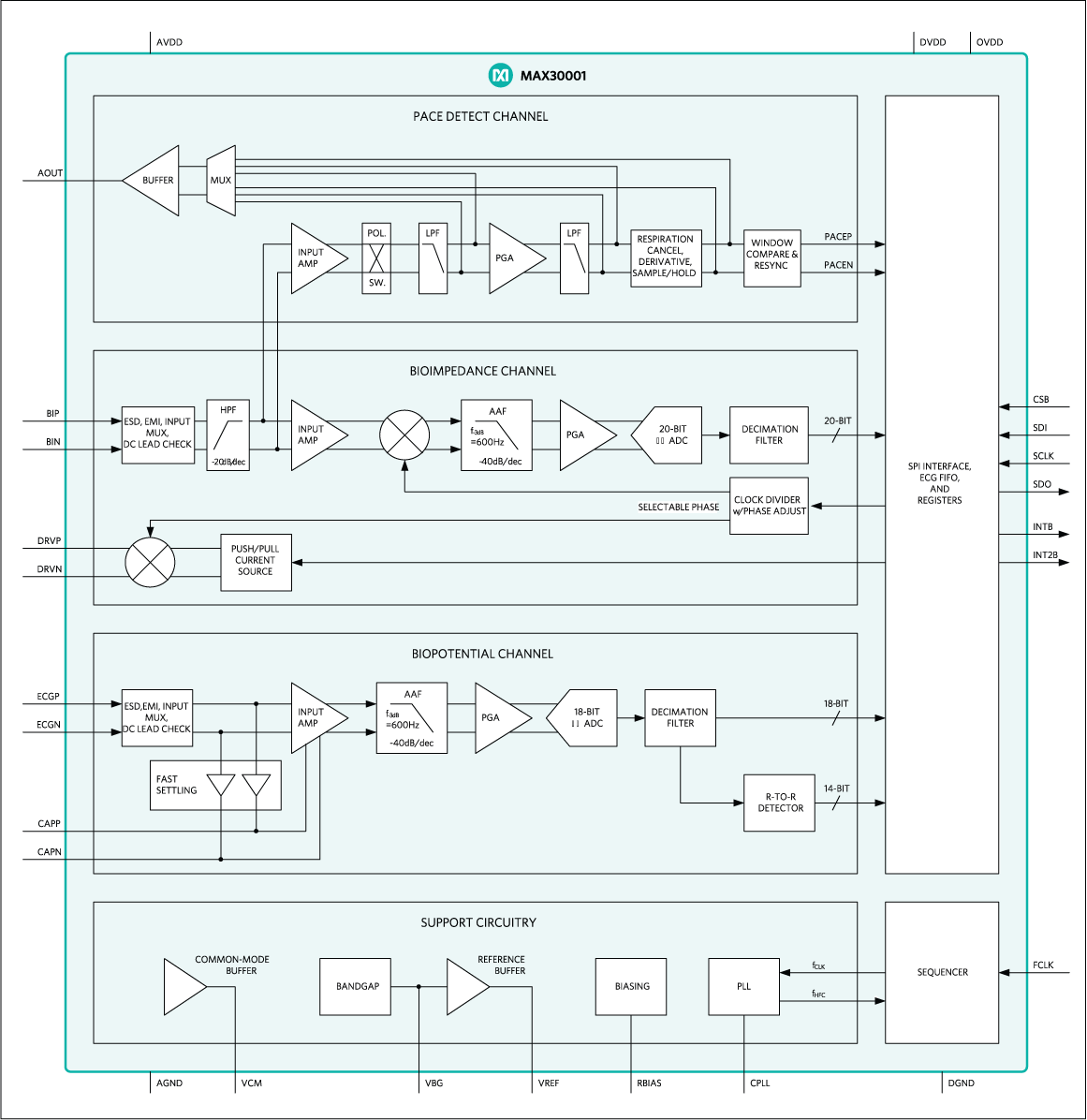
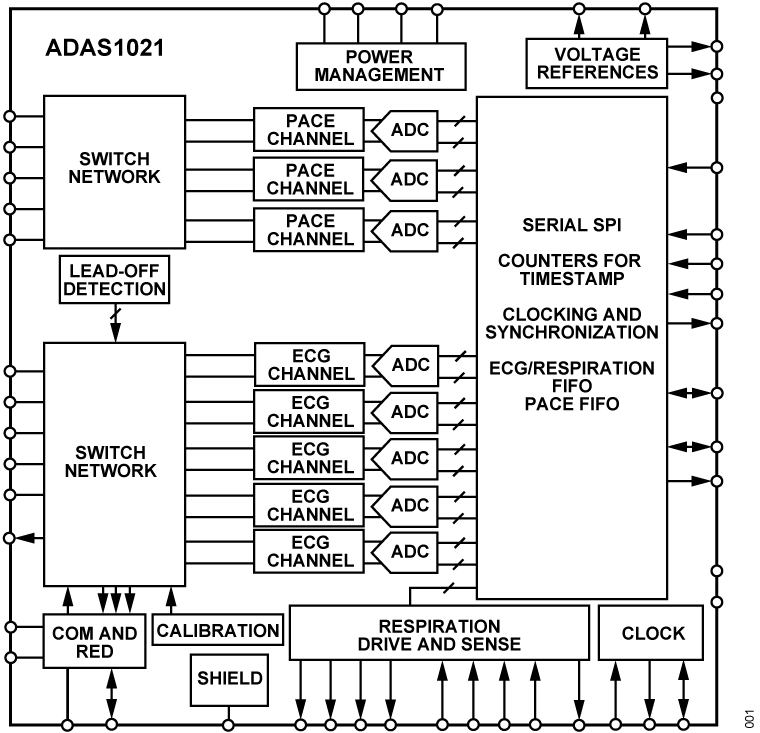
Electrocardiogram (ECG) Measurement Solutions
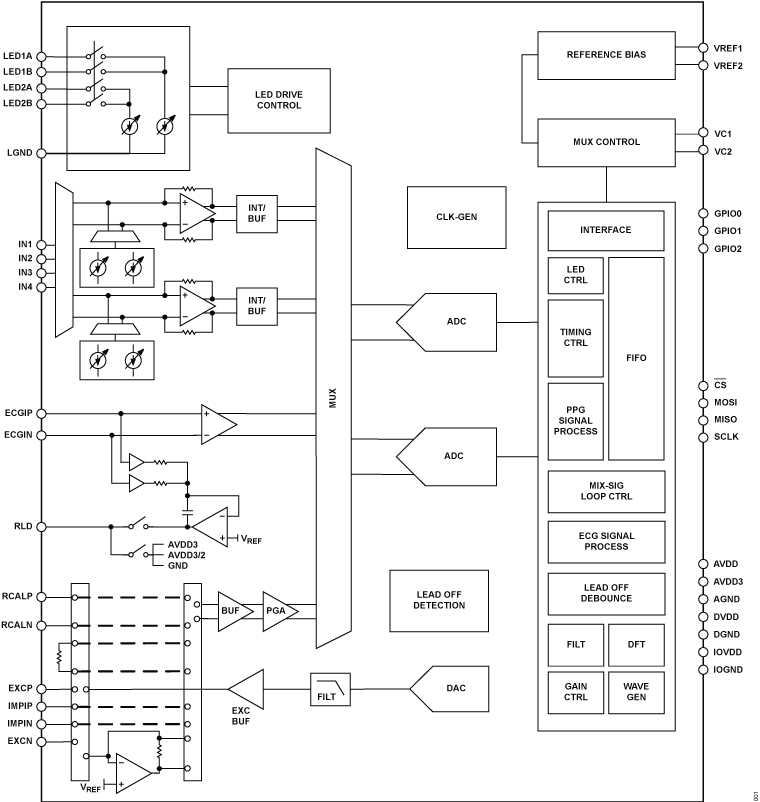
Analog Devices’ electrocardiogram (ECG) measurement application solutions include a wide range of discrete and integrated signal chain products with low-power, low-noise, and multichannel capabilities.
Value and Benefits
For fitness and wearable applications, we deliver low-power, highly integrated solutions with compact analog front ends to accommodate battery-powered use cases.
For clinical applications, we offer multiple lead- and diagnosis-grade performance and analog front ends for superior signal processing capability. ECG measurement not only demands high-precision signal processing, but also accommodates various electrode designs. Our in-house system knowledge ensures best-in-class ECG measurement under different application use cases.


Accommodates battery-powered applications


Features superior signal processing capability


Offers best-in-class ECG measurement under a variety of use cases
Key Resources
Developer Tools and Resources
Design Tools


Simulation Models
Files and Downloads
Book & eBook

Understanding Silent Switcher Technology: High Efficiency, Low EMI eBook
7.75 M
Circuit Note

CN0407: Ultrahigh Sensitivity Femtoampere Measurement Platform
548.59 K

CN0370: 16-Bit, Single-Supply LED Current Driver with Less than ±1 LSB Integral and Differential Nonlinearity
232.9 K
Data Sheet

ADGS1612: SPI Interface, 1 Ω RON, ±5 V, 12 V, 5 V, 3.3 V, Mux Configurable, Quad SPST Switch Data Sheet
373.21 K

ADP5350: Advanced Battery Management PMIC with Inductive Boost LED and Three LDO Regulators Data Sheet
1.68 M
Product Highlight

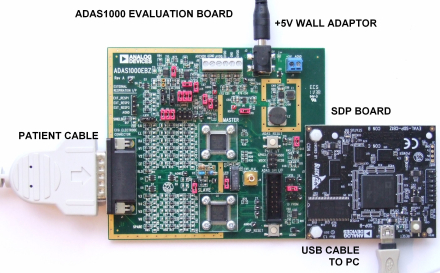
ADAS1000 5-Electrode ECG AFE
595.84 K
Product Selection Guide

Precision Products and Signal Chain Solutions: Selection Guide 2019
13.38 M
Solutions Bulletin & Brochure

Silent Switcher Technology
1.74 M

The Building Blocks of the IoT
1.83 M

Technologies for High Performance Portable Healthcare Devices
598.17 K
User Guide

UG-1091: How to Set Up and Use the ADuCM3027/ADuCM3029
1.67 M
{{modalTitle}}
{{modalDescription}}
{{dropdownTitle}}
- {{defaultSelectedText}} {{#each projectNames}}
- {{name}} {{/each}} {{#if newProjectText}}
-
{{newProjectText}}
{{/if}}
{{newProjectTitle}}
{{projectNameErrorText}}
Featured Products
Interactive Signal Chains