How Common Noise and Error Sources Affect Optical Biosensing
Abstract
Optical sensors represent the most common type of biosensor. This application note examines common noise and error sources that impact optical sensing, including the effects of confounders from the environment captured in the measurement and the physiological variations among the user population. This document also summarizes the current capabilities of wearable biosensors and discusses the future of optical biosensing applications.
PPG Sensors and Environmental Noises
There has been quite a lot of attention on the use of PPG in clinical settings; a pulse oximeter on a finger clip, for example. However, even in the most sterile clinical settings, an optical sensor could capture environmental changes that can affect its optical path(s) and confound the plethysmography information. You can imagine how challenging this becomes in less controlled wearable configurations.
Environmental confounders, or noises, typically fall into two major categories: optical and physiological. Optical noise refers to changing characteristics of the optical path as seen by the sensor that are unrelated to light absorption by the volume of blood observed. For example, an optical sensor can pick up ambient light. This can be particularly troublesome as indoor lighting commonly contains a flicker, which can periodically affect the offset of the sensed optical signal and interfere with the PPG signal. Likewise, a physiological change could alter blood flow and volume in the tissue, which in turn changes the PPG signal.
While such challenges exist in every setting, they become more pronounced in less-controlled environments, like those in which wearable applications are most likely to be found. Even so, PPG remains popular with wearables because the technique has demonstrated itself to be reliable for monitoring key vital signs of the wearers.
To mitigate the impact of some of these artifacts, advanced PPG ICs now have intelligent signal paths. Algorithms, too, have grown more sophisticated. As a result, designers are now able to include PPG in a variety of form factors, including earbuds, rings, necklaces, head and arm bands, bracelets, watches, and smartphones.
Performance Considerations of a PPG Sensing System
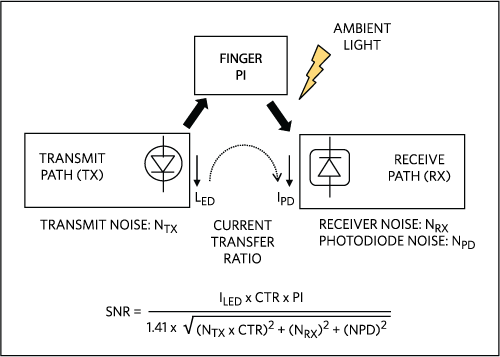
Figure 1. Noise sources in a PPG circuit.
Before discussing optical noises, it is useful to understand the overall performance considerations of a PPG sensing system (see Figure 1). The primary mission of a wearable PPG circuit is to maximize the signal-to-noise ratio (SNR) while conserving expended power.
The perfusion index (PI) represents the ratio of the pulsatile blood flow to the static (non-pulsatile) blood in the tissue. Mathematically, it is the AC portion of the PPG signal as a fraction of the overall signal.
A key contributor to noise and power on the transmit path are the LED drivers, which control the magnitude, transients, and rise and fall times of the LED currents. On the receive path, PPG circuits handle anti-aliasing, sampling, and ambient light rejection. These circuits also maintain power efficiency and, increasingly, signal linearity over a wide sensing range.
An integrated PPG sensor front-end circuit, such as the MAX30112, simplifies the implementation considerations for PPG by combining these functions into a single, cost-effective IC. ICs such as the MAX30112 drive the LED light sources and sample the resulting photodetector output. Based on the selection of LEDs and photodetectors, the photocurrent involved ranges from sub-nA to tens of µAs.
Impact of Ambient Light Conditions on PPG
Both DC and AC ambient light conditions could be problematic for PPG. A strong constant (DC) ambient light can saturate the photodetector, which renders the PPG waveform undetectable. Given this, the front-end circuit must capture the ambient light level when the LED is off and subtract it from the photodetector output before sampling for the PPG signal, as illustrated in Figure 2. In that conceptual drawing, to avoid saturating the converter, a coarse DC signal is removed prior to sampling, and sampling and filter techniques address other ambient light artifacts. After the ambient light component has been removed, the PPG signal can be sampled without the risk of saturation.
Flickers in lighting (mostly indoor) present another noise source for PPG. Based on where they are in the world, indoor lights may flicker with fundamental frequencies at 50Hz or 60Hz. This rate is close to the frequency at which PPG signals are sampled. Left uncorrected, ambient flicker can produce a different bias offset for each sample. Featuring advanced correlated sampling techniques designed specifically to attenuate any 50Hz/60Hz flickering components, the MAX30112 can mitigate the corruptive effects of flickering on the PPG signal.
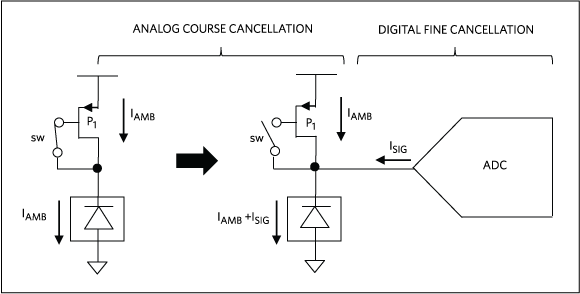
Figure 2. Conceptual drawing showing a two-stage filtering for ambient cancellation in PPG signals.
How Motion Artifacts Affect PPG Accuracy
Some PPG-derived information, such as pulse oximetry (SpO2), is particularly at risk of corruption by motion artifacts. SpO2 is measured by comparing the light absorption of oxy-hemoglobin and deoxy-hemoglobin using a ratio of ratios, R, as follows:

where PI1 and PI2 are the perfusion indices of the system taken using two differently colored lights at wavelengths 1 and 2.

with

where l is the optical path length, ed and eo are the absorption coefficients of deoxy- and oxy-hemoglobin for wavelengths 1 and 2, respectively. In most implementations, the constants (k1, k2, k3, and k4) for a given PPG design are calibrated using known SpO2 measurements from a population of test subjects.
Because the ratios R and PI figure prominently in the equation, the accuracy of SpO2 depends on the ability to maintain consistent PI values. The optical/mechanical design of the PPG probe or wearable, as well as physiological conditions of the subject, all affect PI. When a subject physically moves, either voluntarily or involuntarily, this movement could cause mechanical displacements of the sensor with respect to the tissue. This, in turn, could dynamically modify the efficiency of optical coupling, changing the optical path lengths and otherwise inducing spurious signal dynamics. Even minute movements can affect PPG signals. For example, respiratory motions are routinely coupled into PPG waveforms.
Motion could also cause physiological changes of the tissue that are unrelated to arterial pulses. For example, when a subject changes his or her posture, the movement could partially disrupt blood flow and dynamically redistribute venous blood volume. The change would be reflected in the PPG measurement and, within the context of pulse oximetry, could be interpreted as erroneous. Physiological changes could also occur without motion, such as when there are significant changes in ambient or skin temperature or in hydration. All of these factors can impact PPG observations.
Quite a lot of research has gone into finding ways to improve the accuracy of pulse-oximetry measurements by mitigating the impact of motion artifacts. Algorithms have employed different techniques, from using simple moving averages to complex non-linear adaptive filters. External references can help to qualify the motion artifacts. These references include inertial sensors that would be sensitive to motion but not to changes in optical environment, as well as a light at a third wavelength that would be sensitive to changes in the optical environment but not to motion. Despite the algorithmic advances, however, motion artifacts continue to limit the accuracy of wearable PPG devices.
Wearable Optical Biosensors: New Applications
Even with limitations in accuracy, PPGs have enjoyed success in wearable applications because, in a non-invasive way, they deliver longitudinal, vital sign information, including heart rate, pulse rate, and pulse oximetry. In addition, using PPG signals, advanced algorithms have measured heart rate variability and blood pressure.
With access to a continuous stream of wellness information, we could see a transformation in how we approach healthcare. Insights gained from the information could potentially inform a holistic diagnosis, sometimes before clinically observable symptoms occur.
Indeed, wearable PPGs are advancing far beyond merely reporting vital signs. With PPG data as inputs to data fusion and machine learning, early studies at Standard University used daily wearable biosensor measurements including heart rate, skin temperature, SpO2, and physical activity on 43 individuals to demonstrate that wearable sensors could be useful in identifying the onset of Lyme disease and inflammation. From these observations, researchers have developed computational algorithms for personalized disease detection. To be sure, exciting and ambitious applications are in store for the future.
参考电路
- M J Hayes. P R Smith, A New Method for Pulse Oximetry Possessing Inherent Insensitivity to Artifact, IEEE Trans. Biomedical Engineering, 2001.
- M R Ram, K V Madhav, E H Krishna, N R Komalla, K A Reddy, A Novel Approach for Motion Artifact Reduction inPPG Signals Based on AS-LMS Adaptive Filter, IEEE Trans. Instrumentation and Measurements, 2012.
- I Chen, Personal wellness monitors advance personalized healthcare, NXP.com, 2016.
- X Li, J Dunn, D Salins, G Zhou, W Zhou, S M Schusler-Fiorenza Rose, D Perelman, E Colbert, R Runge, S Rego, R Sonecha, S Datta, T McLaughlin, M P Snyder, Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information, PLOS Biology, 2017.
- S Mukherjee, Can a Wearable Fitness Device Predict Your Heart Attack?, Fortune.com, 2016.
A similar version of this application note appeared on EDN on October 26, 2017.